
High Pressure Freezing – An Emerging Technology in Food Technology
Abstract ::
In the last decade, high-pressure freezing has drawn the attention of many food researchers, mainly due to its potential for improving the kinetics of freezing and the characteristics of the ice crystals formed. Here the different types of high pressure freezing process are explained like the High-pressure assisted freezing (HPAF), High-pressure shift freezing (HPSF) and High-pressure induced freezing (HPIF). Also the applications of High pressure freezing in food industry like in microbial inactivation, preservation of living cells, preservation of sea food, inactivation of enzymes, preservation of gels, preservation of vegetables, etc. are explained in detail. Also the various future scope of this technology is stated here.
1.0 Introduction ::
Freezing is an excellent food preserving method that delays or prevents microbial, chemical and physical alterations by reducing water activity, microbial growth and reaction velocities in enzymatic systems by the formation of ice crystals. In slow Freezing very few ice nuclei are formed leading to very large sized ice crystals while in very fast freezing rate many ice nuclei will be formed limiting the final crystal size. Slow freezing generally results in extensive mechanical damage and reduces the food quality. Water in foods form ice crystals which will break down the cell structure of some foods. This affects the texture and causes mushiness. Thus, in freezing the fast freezing is mostly preferred.
2.0 Principles of High-Pressure Freezing Process ::
From the freezing graph, we can see that different polymorphs of ice are formed during freezing at different temperatures and pressure coordinates. Ice I, the common ice at atmospheric pressure, is stable up to 210MPa (Bridgman, 1912). The freezing point of water decreases with pressure up to 210MPa. The opposite is observed above this level for ice types other than ice I.
Liquid water is denser than ice I. This increase in volume upon freezing is mainly responsible for damage to biological systems when frozen. On the other hand, the volume increment in liquid ice III, liquid-ice V or liquid-ice VI phase changes is negative; also, the phase change enthalpy increment is negative and the slope of the melting curve is positive. We may therefore expect less damage to these ice polymorphs upon freezing.
Super cooling or undercooking, seems to be enhanced by pressure. The homogeneous nucleation temperature of water decreases even more than the melting temperature with pressure up to 210MPa. The meta stable range is thus significantly enlarged with the application of pressure. The minimum temperature at which it is possible to find super cooled water is therefore reduced from -40°C at atmospheric pressure to -92°C at about 210MPa (Ludermann, 1994).
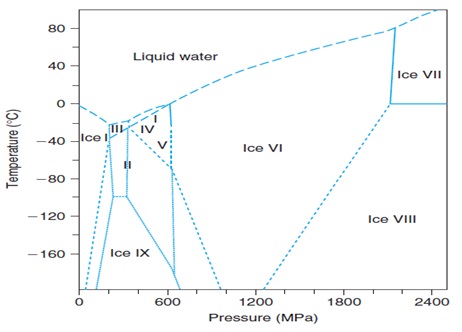
Figure - Triple point of water
3.0 Operational Process of High Pressure Freezing ::
High-pressure freezing processes operate in pressure-resistant vessels with thermally isolated thermostatic circuits to reach temperatures below 0°C. Packed foods are immersed in the pressure/cooling medium and frozen. The apparatus must be equipped with thermocouples to monitor the evolution of temperature during the process in both the product and the pressure medium and also at least with a pressure gauge to measure pressure in the circuit.
Different pressure mediums like silicon oil, propylene glycol, castor oil, ethanol, water, ethylene glycol, etc are used. When choosing a pressure medium, it is necessary to take into account the freezing point of the fluid under pressure, its viscosity and other thermo physical properties (heat capacity, thermal expansion coefficient and specific volume) that influence pressure-induced temperature changes.
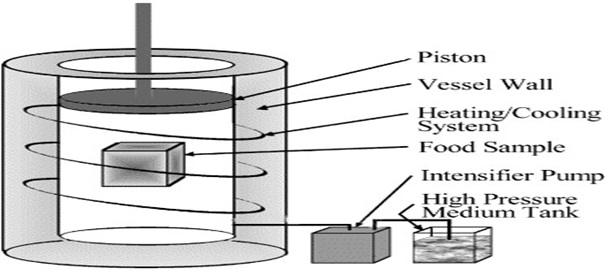
Figure: High Pressure Processing apparatus
4.0 Types of High-Pressure Freezing Process ::
- High-pressure assisted freezing (HPAF)
- High-pressure shift freezing (HPSF)
- High-pressure induced freezing (HPIF)
Here the Phase transition occurs under constant pressure, which is higher than the atmospheric pressure, while the temperature is lowered to below the corresponding freezing point.
First at the normal temperature, the pressure is build up to the desired pressure. Then once the desired pressure is reached, the pressure is kept constant and the temperature is decreased to the freezing temperature. During this decrease in temperature, the phase change occurs. Then at this temperature the pressure is released.
Mostly in this the ice form I or ice form III is formed.
>4.2 High Pressure Shift Freezing (HPSF) ::
The sample is first cooled under pressure below 0°C, but kept in the liquid state according to the phase diagram of water. Once the desired temperature is reached, pressure is released either slowly over several minutes or quickly in a matter of seconds, inducing uniform super cooling throughout the sample due to the isostatic nature of pressure. Due to the pressure release, the phase change occurs and the ice crystals are formed. The faster the pressure release, the lower is the nucleation pressure and the greater the degree of super cooling caused.
When HPSF sample was observed from electron microscope or light microscopy, it showed small ice crystals of granular shape, without specific orientation, dispersed throughout the sample, demonstrating that ice nucleation have occurred throughout the product and not only on the surface. This is widely used in food processing industries.
4.3 High Pressure Induced Freezing (HPIF) ::
This is very recent one, so there is no data of application in food industry.
Here the sample is first cooled under pressure and the phase change is induced by a subsequent pressure increase. In other words, first the pressure is build up, which is lower than the desired pressure. But during this the phase change must not occur. Then at this constant pressure, the temperature is decreased up to the desired freezing temperature. After the desired temperature is reached, the pressure is again build up to the desired pressure and in this process, the phase change occurs.
5.0 Advantages of High-pressure freezing process ::
- Reduced time duration required for the phase change.
- Less mechanical stress during the formation of ice crystals.
- Homogeneous super cooling throughout the sample.
- Formation of small nuclei when latent heat is removed. Thus, less damage to the product.
- An enhancement of product safety can be expected as under synergetic inactivation effects on enzymes and microorganisms occurs during high pressure processes at subzero temperatures.
- High operational cost as compared to the conventional freezing process.
- Transferring this technology to an industrial scale is a challenge, since continuous storage under high pressure at low temperature seems to be a cost-intensive technology.
- Loss of certain nutrients can occur during the prolong freezing. Like at high pressure the protein denaturation can occur.
- Whitening and lightening of colour in certain products like beef and porks were observed.
7.1 Inactivation of microbes ::
Microbes are the one of the main cause of food spoilage now days. Maximum spoilage of the food occurs due to the microbial growth only. Thus, it is necessary to inactivate the microbes in the food to maintain the quality and to increase the shelf life of the food.
Picart et al. (2004) reported on inactivation of Pseudomonas fluorescens, Micrococcus luteus and Listeria innocua inoculated in smoked salmon fish after different pressure treatments. Good results were obtained for pressure shift freezing with slow expansion (cooling at 207MPa for 23 minutes and pressure release in 18 minutes from 207MPa/-21°C followed by further freezing to -25°C at 0.1MPa). It was also reported that reductions of 4.6, 2.5 and 2 log cycles for Psedomonas fluorescens, Micrococcus luteus and Listeria innocua, were noted, respectively.
Thus, the sample can be freezed with the inactivation of microbes, so that even after thawing the microbes does not become active and spoilage does not occur.
7.2 Inactivation of Enzymes ::
Many enzymatic reactions cause many types of spoilage in the food. These enzymes are to be inactivated in order to inactivate their process.
Indrawati et al. (1998) made two enzyme solutions, first solution was made up of polyphenol oxidase and methyl esterase, while the second solution was made up of α-amylases, peroxidase and lipoxygenase. Solution one was subjected to high-pressure shift freezing (200–400MPa, 22/10°C) and solution two to pressure assisted freezing (350MPa/22°C and 400MPa/22°C). the results showed that in solution one Polyphenoloxidase was not inactivated and pectin methyl esterase was only inactivated slightly. While in solution two α-amylases and peroxidase were slightly and reversibly inactivated and lipoxygenase was irreversibly inactivated.
Also in other study carried out for potatoes In high-pressure shift freezing (120, 150, 180, 210MPa/8, 12, 16 and 20°C) indicated that peroxidase and polyphenoloxidase were not completely inactivated and that peroxidase was more resistant to treatment.
Thus, it can be concluded that peroxidase was more resistant to high pressure freezing and so other enzymes except this can be inactivated by this method.
7.3 Preservation of Living Cells ::
Certain living cells are to be preserved for the further ananlysis of the cells or for the study in them. They can also be preserved for their incorporation in certain products for their manufacturing like the yoghurt, curd, probiotics, etc
Galen et. al. (2003) conducted E. coli studies that pressurization rates up to 1.4 kbar/min had negligible effects relative to exposures of >5 min at high pressures. Survivability increased from < 1% at 5 min exposure to 2.1 kbar of pressure to typical values >20%. The combination of glycine and the buffer salt led to even further improvements in survivability.
7.4 Preservation of Sea Food ::
Sea food is the highly nutritious food. It is rich source of certain minerals, proteins and vitamins. But it is highly perishable. Thus, its preservation is necessary.
Dominique et. al. (2001) conducted study on Turbot fish. Turbot fillets were frozen by pressure shift freezing (PSF), 140 MPa, -14oC and then stored at -20oC for 75 days. Smaller and more regular intracellular ice crystals were observed and Ice crystals area in PSF samples was approximately 10 times smaller than that of conventional freezed samples.
Tironi V., et. al. (2010) studied the effect of Pressure Shift Freezing (PSF) on sea bass muscles. The sample was frozen to -15°C to -25°C temperature and 200 MPa pressure. There was no protein denaturation. Extractability, water holding capacity, colour, etc were same to unfreezed sample. Improvement in microstructure was seen as compared to the unfreezed sample.
7.5 Preservation of Meat ::
Meat forms the staple diet of many people in many countries. It being highly nutritious and perishable, the good methods for its preservation is necessary.
Pedro et. al. (2007) studied the effect on beef meat. High pressure processing (20oC or -35oC) was very effective reducing aerobic total (2-log10 cycles) and lactic acid bacteria counts (2.4- log10 cycles), in fresh and salt-added samples respectively. Frozen and pressurized beef stored at -18oC during 45 days recovered its original colour after thawing, similarly to just-treated samples while their counts remain below detection limits during storage.
Realini. C., et. al. (2011) carried out the study for the preservation of Cured Pork Carpaccio. The sample was pressure shift freezed at 400 MPa and 600 MPa at -15°C and -35°C temperature respectively. The results showed that Treated sample had pinker colour, more cooked gel like appearance, low level of Lactic Acid Bacteria (LAB) and psythrotropes. Extended shelf life as compared to the untreated sample.
7.6 Freezing and air cell distribution in dairy foam ::
Volkert M., et. al. (2012) prepared a dairy sample was prepared by the following formulation: Milk Solid non Fat (MSNF) 11.5 % dry matter, sugar 18.0 % dry matter, Fat 9.0% dry matter, Stabilizer 0.5% dry matter and total solids 39% dry matter.
This sample was high pressure shift freezed at 360MPa and -25°C. the air incorporation after this treatment increased to by 78% and the ice crystal size was reduced from 40µm to 34µm. Also there was increase in the mouth feel, texture and smoothness after the treatment as compared to the untreated sample.
7.7 Preservation of Vegetables ::
Luscher et. al. (2005) carried out the study on potatoes. The Potatoes were high pressure shift freezed (HPSF) to ice III at -27oC and 250 MPa. After this the sample was stored up to 24 hour and then the analysis was done. Storage at subzero temperatures without phase transitions resulted in low membrane damage as compared to the other conventional freezing of the potatoes; however, cell lysis was triggered by this treatment.
7.8 Treatment on Gels ::
Martina et. al. (2007) studies the effect of starch gels by high pressure freezing process. The pore size and the total area occupied by the starch gel pores were reduced by high pressure freezing at 150–240 MPa compared with freezing at atmospheric pressure at the same cooling rate.
The pore size in the high-pressure frozen gels did not increase during a storage time of 3 months at −24 °C at atmospheric pressure.
8.0 Conclusion and future aspects ::
The industrial application of this technology requires large optimization of the processes for the maintenance of such temperature and pressure.
More work is still needed to be done in this area of freezing. It is an emerging technology with great advantages in food technology. The freezing effect can be obtained at the temperatures higher than the freezing temperatures. Thus, the energy required for the attainment of such lower temperature can be saved. Also the product quality is good as compared to the normal conventionally freezed sample.
REFERENCES:
***************************************************
Saxena Srishti Rajesh
M.Tech (Food Processing Technology),
Student, Anand Agricultural University,
Anand 388110



Home | Archive | Advisory Committee | Contact us