
Synthesis of Layered Tin Monoselenide Crystals by VT technique
Abstract Compounds consisting of group-IV elements (Ge, Sn and Pb) and group-VI elements (S, Se and Te) are known as narrow gap semiconductors or semimetals which have a potential for various optoelectronics applications. Several such compounds which are not accessible by usual crystal growing methods can be grown by vapour transport technique. The technique is generally preferred due to its relative simplicity and wide applicability. It is particularly suited for high melting compounds or for those which decompose without melting. The use of a transporting agent, usually a halogen, dramatically enhances the growth rate for AIV BVI compounds.
Keywords:Tin monoselenide ,crystals, vapour transport technique
1. Introduction
The AIV – BVI Intermetallic compound SnSe possess interesting semi conducting properties. SnSe belongs to the interesting class of isomorphic materials that are in many ways between two – dimensional (layer type) systems and three- dimensional crystals, and it exhibits a strong anisotropy of optical properties [1,31-32]. SnSe is a semiconductor with a band gap of about 1eV and therefore should possess the potential to work as an efficient solar cell material [2]. The selection of SnSe from orthorhombic IV- VI layered materials is because SnSe is attracting considerable attention recently on account of its semi- conducting nature and applications in infrared optoelectronic devices [3], radiation detectors [4], holographic recording systems [5], electrical switching [6] and polarity dependent memory switching [7]. One of the main causes of the technological difficulties of the growth of the Si, Ge and Sn chalcogenides is the existence of many polytypes and polymorphs of these compounds. For crystal growth one uses very different methods that determine, on one hand, the multi-facetted physico - chemical properties of the semiconducting materials, and, on the other hand, determine the particular method for preparing the material with pre-established properties.
2. Experimental
Several materials which are not accessible by usual crystal growing methods such as modified Czocharalski or Bridgmann – Stockbarger techniques can be prepared by Chemical Vapour Transport technique. It is particularly suited for high melting compounds of for those which decompose without melting.
The nature and quantity of the transporting agent alters the dimension and morphology of the obtain single crystals [29].Chemical Vapour Transport (CVT) technique mainly relies on a chemical reaction between the source material to be crystallized and a transporting agent. The reaction product is volatile and can be transported into the vapour phase at temperature well below the melting point of the compound.
Therefore, the growth of SnSe crystals may be undertaken by Chemical Vapour Transport (CVT) technique.
The method of crystallization with the participation of chemical reactions (method of chemical transport and decomposition or activation of the chemical compounds) was applied for the growth of crystals of materials with low value of the pressure of own vapors below the melting temperature or materials that loss essentially their stoichiometry during sublimation. The growth of crystals by chemical compounds transport is achieved in closed system [30].
The advantage of the method of chemical transport reactions (CTR) over other methods of getting AIV BVI from the vapour phase is the possibility to achieve the growth to much lower temperatures. This allows for getting more perfect and stiochiometric crystals, and also, gives the possibility to synthesize crystals of the low temperature modifications.
The temperature-length profile for the synthesis of SnSe single crystals by Vapour Transport technique is shown in figure1.
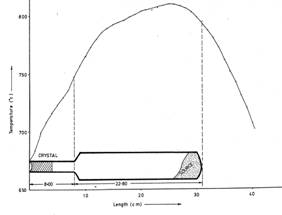
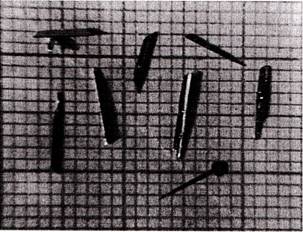
Such thin platelets of layered tin monoselenidesingle crystals grown by chemical vapour transport technique using NH4Cl as a transporting agent is shown in the following figure:
The reagent carrier used here is NH4Cl. The growth velocity of the crystals decreases with the increasing pressure. For enough productive crystallization process, it is not necessary a large amount of transporting agent. Usually in the ampoule NH4Cl is introduced in an amount not higher than 4mg/cm3.
3. Discussions
The methods of Crystal growth, usually are classified after two main characteristics:
1) The phase state and the component of the initial material
2) The driving forces
According to the first principle the following group of methods
- growth from stoichiometric melts
- growth from solutions
- growth from gaseous phase
The classification of the methods in the limit of the above main groups is made after the character of the driving force. The driving force of the growth process are the temperature gradient, pressure, concentration or chemical potentials. Therefore, every known growth method is based on the maintaining during growth of an optimal value of the gradient of one from these parameters, usually the temperature.
Let us see the basic principle of the methods of growth of the layered chalcogenide of the crystals from the IVth group from melt and gaseous phase. The growth of crystals is determined by the conditions of the phase transitions, which ensure the production of single crystals with given composition and properties.
The SnSe bulk single crystals have mainly been grown by the Bridgman method [8, 9], the close – tube – vapor- transport technique [10, 11], the direct vapor transport technique without transporting agent [12], and the solvothermal technique [13]. The growth of the chalcogenide crystals of the IVA subgroup with the sublimation- condensation method was conducted in closed or flowing systems [5,14-27]. A closed system can be obtained in sealed quartz ampoules and Pizzarello method [28].
The shortcomings of the growth method for the AIV BVI crystals from melt are : low velocity of this process, the necessity to use high temperature that lead to the increase of the defect concentration (vacancies and dislocations) in the crystals, the localization in the crystal of all the impurities from the initial material. Therefore, during the development of the technology of crystal growth for AIV BVI and AIV – B2VI compounds there was paid high attention to the methods of their preparation from the vapour phase.
Majority of compounds of the transition metal dichalcogenides belonging to MX2 group are insoluble in water and decompose before their melting points are reached. Therefore, the growth of such crystals from the melt and aqueous solution are not possible. Hence, the growth of single crystals of these compounds from vapor phase technique was found to be most suitable.
The growth of crystals from the vapour phase can be performed at significantly lower temperatures, essentially below the melting temperature of the material. This means that the concentration of vacancies and dislocations in the crystal can be diminished to a minimum (the vacancy concentration depends exponentially on temperature). Apart this, the methods of low temperature crystallization are the most acceptable for getting crystals of materials that melt incongruently and, also, materials with tendency towards polymorphism, when it is necessary to get crystals of low temperature polymorphous modification. The other advantage, due to choosing the method for getting chalcogenide monocrystals of the IVth group, is related to the enough high vapour pressure, therefore the mass transport through the vapour phase is easily achieved.
The growth of crystals AIV BVI from the vapour phase is performed by several methods, which conventionally can be divided in two classes: methods based on pure physical condensation, and methods, that suppose the participation of a chemical reaction whose product is the crystallized material. In the first class, the most important is the method based on the process of sublimation – condensation, and the second class is based on methods of chemical synthesis in the crystallization region on the account of decomposition (or activation) of the gaseous chemical compounds and chemical transport.
The main shortcoming of the growth of layered mono-crystals of the type AIV BVI using the sublimation method consists in the fact that the size of the crystals is not large, as required by the industry. This is due to the low velocity of crystal growth from vapour phase (ten- hundreds parts of millimeter per hour), difficulties of controlling the process of nuclei formation (especially for mass spontaneous crystallization), complications in the stabilization of the growth conditions for long time, etc… Nevertheless, the advantage of this method is the possibility to control the process below the melting temperature (due to high vapour tension of the solid)
Acknowledgement
Authors are hearty grateful to PRINCIPAL Dr N M PATEL for constantly inspiring and reminding us to contribute research paper in e – journals.
REFERENCES :
***************************************************
Ajay Agarwal
Department of Physics, Shree Jayendrapuri Arts & Science College
Bharuch
Department of Physics, Shree Jayendrapuri Arts & Science College
Bharuch
Department of Physics, Shree Jayendrapuri Arts & Science College
Bharuch
K R Chaudhari
Department of Physics, Shree Jayendrapuri Arts & Science College
Bharuch



Home | Archive | Advisory Committee | Contact us