
Powder XRD,FT - IR and Thermal Study of Copper Tartrate Crystals
ABSTRACT
Copper Tartrate crystals were grown by single diffusion gel growth technique. Optimum conditions were established for the growth of good quality crystals. Grown crystals were characterized by powder XRD, FT-IR and TG-DTA-DSC. Grown crystals shows O-H, C=O, C-O, C-H and Metal Oxygen Bonds. Crystals were thermally stable up to 111 oC. Copper tartrate crystals shows orthorhombic crystal system.
[1.0] INTRODUCTION :Tartaric acid, a common byproduct of the wine industry, may serve as a base for the development of new class of materials. The presence of the two hydroxyl as well as two carbonyl groups in tartaric acid permits the ready incorporation of monovalent, divalent or trivalent metal ions as well as incorporation of phosphorus-containing moieties. Mainly tartrate crystals possess application, as dielectric, ferroelectric and piezoelectric materials or show non-linear optical properties [1–3]. These characteristics of Tartrate compounds are utilised for their use in transducers, linear and non-linear mechanical devices [2]. Sodium Tartrate Dihydrate is commonly used as primary standards for methods that determine water contents [4]. Lanthanides form a series of compounds with properties that change regularly with increasing atomic number of lanthanide [5].
For the materials which show poor solubility in water and which decompose before melting and do not vapourise or sublime on heating, the gel method offers an attractive advantage for their synthetic crystallization [6]. Several researchers have grown materials of great interest from both solid state sciences as well as technological point of view in single crystal and polycrystalline form by gel technique [7] and modified such materials by suitable substitution to determine the effect of modification of composition on their characteristics [8–10]. Thermal stability of phosphinated diethyl tartrate which is flame retardant has been reported [11,12].
Some tartrate compounds are used in military applications. Strontium Tartrate is used in ammunition units [13] Manganese tartrate crystal being temperature sensitive can be used to sense and measure the temperature. A wax pencil is developed to sense the surface temperature of heated substance in terms of change in the coloration upon contact. The change in coloration of this crystal occurs at 410oC from pink to black. The coloration change is almost instantaneous and occurring within 1 to 2 seconds. Gvozdov and Erunov [14] described this method. The effect of light on copper tartrate has been examined [15].Copper tartrate has shown in vitro to stimulate Luteinizing hormone. Clomitrol is the drug used to add a specific type of copper to the testosterone regulating mineral complex [16]. The synergist effect of tartaric acid for zinc ion in cosmetics has been reported [17]. Zinc tartrate and zinc tartrate with other compounds form a bright coating and used as protecting powder for metals [18].
In the present investigation, the authors have grown prismatic crystals of copper tartrate. The results obtained by powder X-ray diffraction (XRD) studies, Fourier transform infra-red spectroscopy (FT-IR) and thermogravimetric analysis (TG- DTA-DSC).
[2.0] EXPERIMENTAL :
[2.1] Growth ProcedureThe apparatus used for crystallization of single crystals of pure and modified copper tartrate by gel technique consists of borosilicate glass tubes of length 20cm and diameter 2.5cm placed vertically on a wooden stand. Silica gel was prepared by adding a solution of sodium meta silicate to tartaric acid slowly with continuous stirring to avoid any local ion concentration, which would otherwise cause premature local gelling and make the final solution inhomogeneous. A fixed amount of gel solution with 1.04 g/cm3 specific gravity and value of pH was set at 4.0 by adding 1M solution of tartric acid transferred to several test tubes. The test tubes were sealed with some suitable material to prevent fast evaporation and contamination of the exposed surface of the gel. The gel setting time was found to be strongly dependent on pH and environmental temperature. After confirming the gel setting, an aqueous solution of copper chloride of a particular molarity was poured over the gel carefully along the walls of the test tube so as to avoid any gel breakage. The following reaction is expected to take place leading to the formation of copper tartrate crystals:
CuSO4 .nH2O+H2C4H4O6 ![]() CuC4H4O6.mH2O + H2SO4 + (n-m)H2O
CuC4H4O6.mH2O + H2SO4 + (n-m)H2O
Where,n ≥ m
To grow copper tartrate crystals, copper sulphate solution was poured on the gel. The diffusion of Cu2+ ions through the narrow pores of the silica gel leads to the reaction between these ions and the C4H4O62- ions present in the gel. The reaction leads to the formation of copper tartrate crystals as pet above chemical reaction.
2.1 Characterization Techniques
In the present investigation, an attempt is made to find out the cell parameters of copper tartrate crystals and compare the same with the reported values. Using DEBYE computer programme, h, k and l parameters as well as d and 2q values are generated in such a way that these values match with the X-ray powder diffraction values, then by using the programme REFEDT.BAS. the new values of a, b, and c are generated from the h, k, l and d values obtained earlier. FT-IR spectrum was recorded by using Nicolet (Thermo Scientific) - 6700 set up in the range from 400 cm-1 to 4000 cm-1 in KBr medium. The TGA, DTA, DSC was carried out on LINSEIS STA PT -1600 from room temperature to 900oC at a heating rate of 15 oC/min.
[3.0] RESULT AND DISCUSSION
[3.1] Growth Observations
After adding 1 M CuSO4 supernatant solution on set gel, a 1 cm thick band of small crystals was observed after one day. This band further fattened to 1.7 cm and a few scattered crystals began to grow below it after two days. After four days the band extended to 2.2 cm width, the density of number of crystals decreased on moving towards the bottom. After six days the band increased up to 2.9 cm width and a few large size crystals were grown in the lower region of the band. This band was further swollen to 3.2 cm and 3.6 cm after nine days and eleven days, respectively. After fifteen days it expanded to 3.8 cm and more crystals were found to be growing in the lower part of the test-tube. Some crystals had apparent dimension up to 4 mm. Fig. 1 (a) shows the grown copper tartrate crystals in test tube and Fig. 1 (b) shows the magnified view of crystals in gel. Fig. 2 shows the grown crystals.
(a) (b)
Fig. 1 : Copper Tartrate crystals (a) in test tube (b) magnified view of the grown crystals in gel

Fig. 2 : Grown Copper Tartrate crystals
[3.2] Powder XRD study
Each copper atom forms total of five bonds with three tartrate molecules. A sixth one is formed with water, resulting in distorted octahedral co-ordination geometry. Each copper atom chelates by two tartrate groups and each
tartrate group chelates two copper atoms. A water molecule and a nonchelating carboxy-oxygen atom of another tartrate group complete the coordination around each copper atom. The dimensions of the tartrate group are significantly different from those found in manganese L-tartrate tetra-hydrate.
The present analysis indicates that the cell parameter values are closely matching with the values reported by Soylu[19]. Within the limits of estimated standard deviation, the present values are as follows;
a = 8.3650(14) Å b =12.8350 (13) Å c = 8.7580 (9) Å
This suggests that the structure is of orthorhombic type.
However, an attempt is made to analyze the crystal structure of copper tartrate crystals by considering the triclinic structure suggested by Bridle and Lomer [20]. The values of cell parameters indicate the large number of deviation in standard errors and sigma cannot be minimized appreciably low. The crystal structures determined are as follows;
a = 8.5575(102) Å b =12.4450 (127) Å c = 8.8500 (66) Å
This further suggests that the crystal structure is orthorhombic and the result is much more nearer to the values suggested by Soylu[19] and the triclinic crystal structure is ruled out hereby. Fig. 3 shows the powder XRD pattern of copper tartrate crystals.
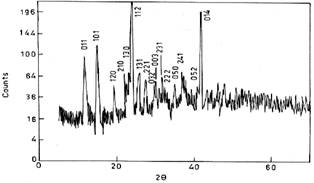
Fig. 3: Powder XRD pattern of copper tartrate crystals
[3.3] FT-IR Spectroscopic studies :
FT-IR Study was carried out to study the chemical bonding present in the material. FTIR spectroscopy has been applied for probing the growth of a crystal organic template. Directed mineralization of calcite crystals has been probed in situ by external reflection absorption FTIR spectroscopy in an effect to understand the dynamics of the organic-inorganic interface during crystal growth. The nucleation face type of calcite has been visually identified according to the known crystal morphologies and corresponding carbonate stretching and deformation vibration bands.
Organic template nucleated calcite at (010), (001) and (012) planes have been studied by Ahn et al. [21]. This suggests novel application of FTIR spectroscopic technique in crystal growth.
Sheveheko [22] studied the IR spectra of both normal and partially deuterated compounds of some tartrates and found absorptions at 600cm-1 and 400cm -1 due to COO¯ group in metal tartrates. Fig. 4 shows FT-IR spectrum of copper tartrate crystals.
Fig. 4: FT-IR spectrum of copper tartrate crystals
It can be observed from fig. 4 that the the O – H stretching is clearly observed in the form absorption around 3260 cm-1. The C = O stretching due to carbonyl group is observed around 1600 cm-1. The O – H out of plane bending is seen around 1050 cm-1. The C – O stretching vibration is observed around 1380 cm-1, 1236 cm-1. The C – H stretching is also observed around 1103 cm-1. Presence of Metal Oxygen bonding is clearly shown from the absorption between 900 cm-1to 450 cm-1.
[3.4] TG-DTA-DSC
Thermogravimetric analysis (TG) is a very useful technique to assess the thermal stability of various substances. Many authors have reported TGA for various tartrate crystals. Selvarajan et al. [23] studied the thermal properties of calcium tartrate single crystals using TGA. Thermal properties of gel grown pure and nickel doped strontium tartrate tetrahydrate single crystals were studied by Rethinam et al. [24]. Ramkrishnan [25] reported TGA studies on manganese tartrate trihydrate crystals, whereas, the thermal study on rare earth pure and mixed tartrate crystals was reported by Kotru et al.[26-27]. Moreover, the TGA study was reported on iron (II) tartrate crystals by Joseph et al. [28] and on calcium tartrate crystals by Joshi and Joshi [29]. Recently, thermal decompositions of Bi (III), Cd (II), Pb(II) and Cu (II), Thiocynates have been reported by Ptaszynski et al.[25]
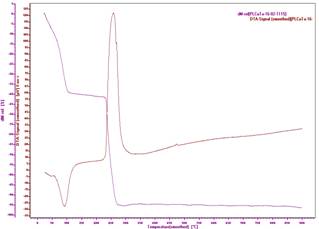
Fig. 5: TG – DTA curve of copper tartrate crystals
Fig. 5 shows the decomposition of hydrated copper tartrate crystals. At 111oC hydrated copper tartrate crystals first decompose into anhydrous form. After that it decomposes every slowly and then a sharp decomposition takes place around 232oC and CuO is formed at 275oC. The DTA – DSC peak shows that first decomposition in the form of endothermic reaction is observed at 90.1oC. This shows the dehydration of copper tartrate. Thermodynamic parameters for this reaction are Enthalpy, Delta Cp (change in heat capacity) and Heat Change are -668.90 J/g, 3.7405 J/gK and -269.35 μVs/mg. An Exothermic reaction is observed around 258oC, which may be due to formation of gaseous products from copper tartrate. Thermodynamic parameters for this reaction are Enthalpy, Delta Cp and Heat Change are 2669.47 J/g, 1.8709 J/gK and 1217.97 μVs/mg.
[4.0] CONCLUSION :
Bluish prismatic crystals were grown by single diffusion gel growth technique. Gel pH was set at 4.0 by adding appropriate amount of tartaric acid in the solution of sodium meta silicate. After setting the gel solution of copper chloride was poured on set gel, after 15 days full grown crystals were obtained inside this gel. Powder XRD confirms the orthorhombic crystal system of the grown crystals. FT-IR confirms the O-H, C=O, C-O, C-H and Metal Oxygen bonds. TG shows that the crystals remains stable up to 111 oC and DTA study shows an endothermic reaction at 90.1oC and an exothermic reaction at 258oC.
Acknowledgement
Authors are thankful to their Ph.D. supervisor Prof. M. J. Joshi, Head, Physics Department, Saurashtra University for his useful guidance through out their work. Authors RMD and PMV also thankful to the principals and the managements of S. V. Virani High School and M & N Virani Science College, respectively, for their keen interest and encouragements.
REFERENCES :
***************************************************
R. M. Dabhi
S. V. Virani High School,
Rajkot
M & N Virani Science College,
Rajkot



Home | Archive | Advisory Committee | Contact us